Designer Genes: Genetic Engineering on a Changing Planet
October 17, 2025By Nethanya Fonseka, 2025 Future Blue Youth Council member
Cover photo: Baroque Biology (Paper Theatre) by Jennifer Willet (2019), petri dishes, agar, GMO bacteria, collage materials | Credits: papertheatre – J.Willet
Scientists have been at the helm of discovery for generations. Every breakthrough pulls back another layer of the universe above us, the world around us, and the life within us. Although humanity’s scientific pursuits have improved our understanding, communication, and quality of life, significant environmental, societal, and global challenges remain. In the face of uncertainty and struggle, we must continue our search for solutions to sustain ourselves and our planet.
One solution that offers both promise and controversy is genetic engineering. Genetic engineering is the process of directly altering the DNA of an organism, often to give it new traits or remove unwanted ones. Through inserting, deleting, or modifying genes, scientists can influence how an organism functions.20 Genetic engineering can lead to healthier ecosystems, greater food security, and increased carbon capture, but it also raises serious ethical and ecological concerns. We are talking about life itself here.
Before exploring the good, bad, and ugly of genetic engineering, allow me to briefly explain how it works.
Agrobacterium-mediated transformation and biolistic transformation are genetic engineering techniques that deliver DNA into cells, while CRISPR, TALENs, and homologous recombination are methods that directly edit genes.6 The most modern gene-editing technology is CRISPR-Cas9, which scientists adapted from a natural bacterial immune system that recognizes and cuts viral DNA.15
When using CRISPR, scientists begin by identifying the DNA sequence they want to study or modify. Then, they create a gRNA molecule that matches the target sequence through chemical synthesis or transcription from a DNA template.10 Scientists then use biotechnology tools and techniques to produce Cas9–the enzyme that will cut the target DNA.24 Using test tubes or microplates under controlled conditions, scientists allow the gRNA and Cas9 to bind and form a stable complex, and deliver it into the cell using methods like electroporation, microinjection, and lipid nanoparticles.12
Once inside, fascinating things unfold at the molecular level. First, the gRNA searches the cell for its matching sequence. Once found, Cas9 binds to the DNA and cuts both strands at the target location.24 The cell will naturally try to repair itself, but the process is highly error-prone and can disrupt the gene’s function.14 If disabling the gene was the scientists’ aim, the process is complete.
However, scientists often want to correct or change a gene. To do so, they would provide a DNA template and use delivery methods such as liposomes and lipid nanoparticles to get it inside the cell.23 When the cell detects the break, it will use the inserted DNA to repair the cut.8
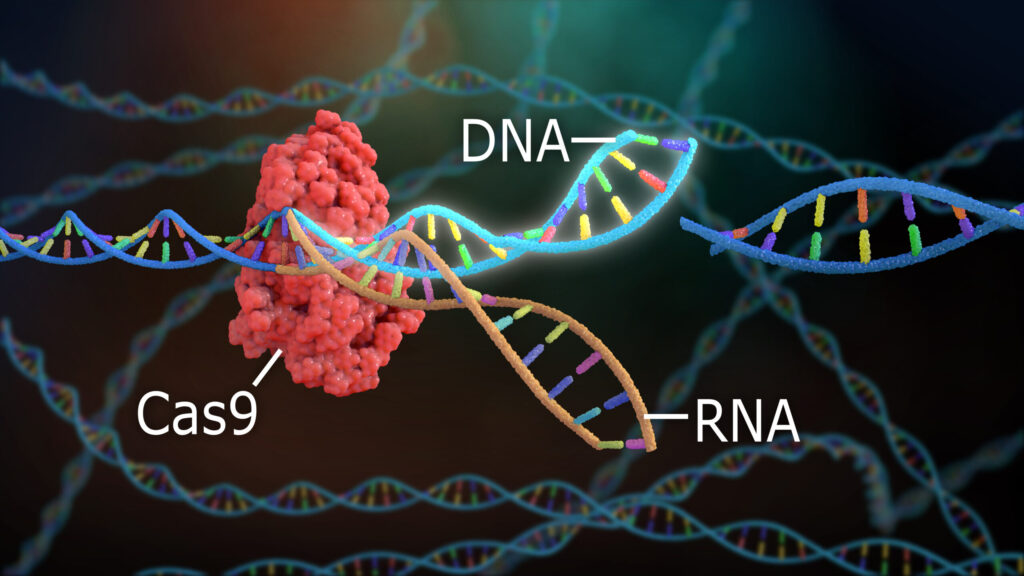 CRISPR Visualization. Image credit: Nathan Devery/Shutterstock.com
CRISPR Visualization. Image credit: Nathan Devery/Shutterstock.com
This is an oversimplified rundown of CRISPR. For further demystification, check out the Innovative Genomics Institute’s resources. And remember, like every other technology, CRISPR has its limitations; however, scientists are constantly researching ways to make it more precise and versatile.21
Now, let’s discuss how genetic engineering is used in an ecological context.
Throughout the 1900s, insect pests caused major damage to crop yields around the world. Farmers became increasingly reliant on chemical insecticides, many of which were environmentally and economically unsustainable.16 Scientists recognized the need for improved pest control, and turned to the soil bacterium Bacillus thuringiensis (Bt), which produces insecticidal proteins that target certain insect pests but are non-toxic to humans, vertebrates, and most beneficial organisms.1
Researchers introduced modified Bt genes into crops using biolistic transformation (gene guns) and Agrobacterium-mediated transformation, creating Bt corn and Bt cotton.1 These genetically engineered crops can produce their own Bt proteins, resisting pests and improving crop yields, while also reducing the need for chemical insecticides.
Bt crops have been adopted in over twenty developed and developing countries across six continents.3 Moreover, there have been recorded regional decreases in European corn borer and corn earworm populations in areas with widespread Bt corn adoption.4 For growers in Illinois, Minnesota, and Wisconsin, cumulative benefits over 14 years for Bt corn use are an estimated $3.2 billion.7 In India, Bt cotton has caused a 24% increase in cotton yield per acre and a 50% gain in cotton profit among smallholders due to reduced pest damage and reliance on insecticides.9
 Bt Corn Fall Armyworm Resistance. Source
Bt Corn Fall Armyworm Resistance. Source
Despite the benefits of Bt crops, significant challenges remain. Certain populations from five of the target pests have evolved resistance to Bt traits, jeopardizing crops and those who rely on them for sustenance and income.3 In response, scientists are closely monitoring pest populations and managing resistance through strategies like refuge planting, crop rotation, and stacking multiple Bt traits.1 Many wonder if Bt crops will harm other organisms; however, extensive laboratory and field studies have not found evidence of significant adverse effects on non-pests.18 Overall, Bt crops demonstrate how genetic engineering can be used and improved to increase crop yields, reduce pesticide use, and combat agricultural problems.
Scientists are also using genetic engineering to enhance the natural bioremediation abilities of microorganisms. Soil-associated bacteria and plants can naturally degrade pollutants from industrial waste and chemical runoff; however, their abilities are often limited and slow.17 With pollution threatening wildlife and societies worldwide, scientists have genetically modified bacteria to more effectively detoxify pollutants like chemicals, xenobiotics, and pesticides under experimental conditions.17
Microorganisms such as bacteria, fungi, and archaea rely on enzyme-catalyzed reactions to degrade pollutants.17 However, many harmful contaminants resist bioremediation and consequently accumulate in the environment.17 To address this issue, scientists have used gene-editing tools like CRISPR to improve the abilities of bacterial strains to perform reactions and remove pollutants.17
In an environmental strain of Pseudomonas aeruginosa, scientists used CRISPR to disable the rpoS gene, enhancing its ability to break down gasoline.19 In Streptomyces coelicolor M145, scientists overexpressed the alkB gene, enabling it to digest the pollutant n-hexadecane with greater efficiency.17 In Acinetobacter sp. BS3, scientists cloned and inserted the C230 gene, improving its degradation of pollutants from crude oil.25 In Deinococcus radiodurans, scientists inserted the merA gene from Escherichia coli, enabling it to reduce toxic ionic mercury into less harmful elemental mercury.2 The genetically-engineered strain of D. radiodurans can also break down mercury and toluene in highly radioactive environments.17 Research indicates that bioengineered strains are better suited for extreme survival than wild types, making them incredibly valuable in ridding our ecosystems of radioactive pollutants.17
These examples reveal how genetically modified bacteria can more effectively degrade pollutants and thrive in challenging conditions. However, only a few strains are actively detoxifying real-world environments, so we must consider how introducing them will affect existing microbial communities and their natural synergism in degrading pollutants.17 While this technology requires more research and trials, it offers great promise in reducing pollution and protecting our environment.
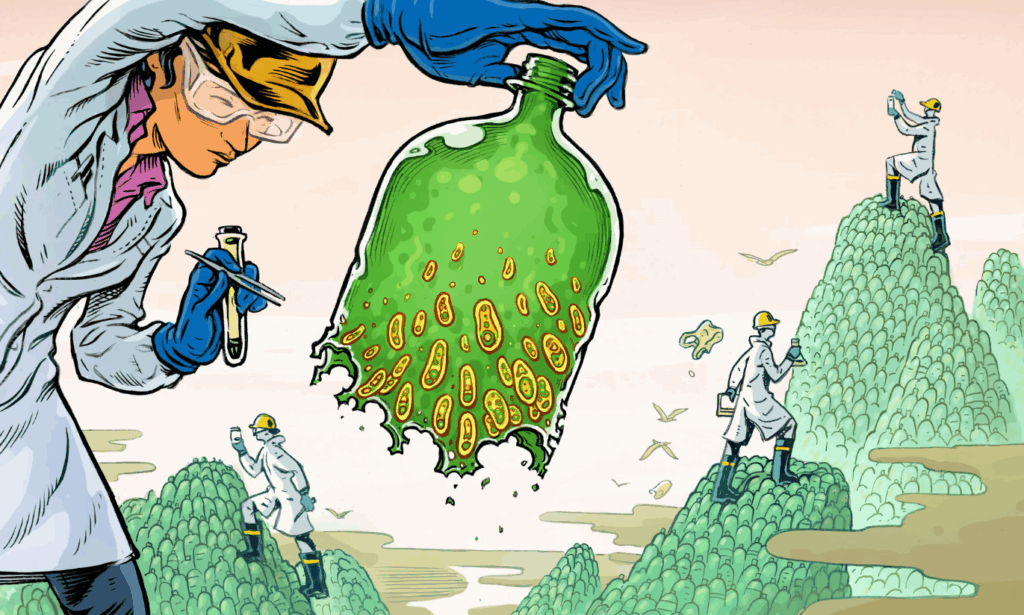 Pollution-degrading Bacteria. Image credit: Lars Leetaru/The Guardian
Pollution-degrading Bacteria. Image credit: Lars Leetaru/The Guardian
As we all know, there are a plethora of environmental challenges we face today. As such, there are a plethora of potential uses for genetic engineering. Scientists are using CRISPR to identify the genes responsible for heat-tolerance in coral on the Great Barrier Reef.5 Biotech company Living Carbon is using biolistic transformation to create poplar trees that can grow faster and sequester more CO₂.22 Scientists are researching how gene-editing can improve nitrogen usage in plants and reduce the need for chemical fertilizers.11 These are some of genetic engineering’s many applications and solutions–all of which are ultimately aimed at supporting the natural world and its inhabitants.
Proponents of genetic engineering argue it gives us solutions to meet urgent environmental challenges. Crops can be modified to survive in harsh or new conditions. Engineered microbes can help clean up toxic waste. Enhanced trees can capture more CO₂. When done carefully, biotechnology can safeguard communities and ecosystems from the massive, daunting problems we face today. The overall argument is that genetic engineering, when used responsibly, can be one part of our solution toolkit.
Opponents of genetic engineering fear unintended ecological consequences. We can’t afford to experiment with the wild. We don’t have a “backup Earth” if things go wrong. The smallest mistakes can still cascade through entire ecosystems. There are also societal and ethical considerations–Do GMOs have adverse impacts on human health? Is it ethical to change the genome of any organism? Some argue that genetic engineering is a shortcut. Shouldn’t we be spending our time, resources, and money on conserving and protecting what we already have? Should we modify corn because of the changing environment, or should we prevent the environment from changing in the first place?
Companies like Monsanto have undermined public trust and support in genetic engineering–not because the science is evil–but because of how it’s applied and managed. We have to remain informed of how the technology is used, who controls it, and what it leads to. These questions and answers matter just as much as the genes themselves.
In conclusion, genetic engineering has good, bad, and even ugly sides. We have to remember that science is progress, but it’s also responsibility. Every breakthrough and issue has many sides, and every voice in the discourse matters. In the end, the choices we make about genetic engineering and about so many other societal and environmental issues are choices about how we sustain ourselves and our planet. There are no simple answers, and the road will be filled with dilemmas, disagreements, and even discord.
But walking it is a shared responsibility.
We will have to decide how to walk it wisely–and I hope it’s together. We will have to consider all creatures and communities in the decision-making process. We will have to always remain mindful of the power and responsibility that comes with designer genes.
Bibliography
- Abbas, Mohamed Samir Tawfik. “Genetically Engineered (Modified) Crops (Bacillus Thuringiensis Crops) and the World Controversy on Their Safety.” Egyptian Journal of Biological Pest Control 28, no. 52 (June 19, 2018). https://doi.org/10.1186/s41938-018-0051-2.
- Brim, Hassan, Sara C. McFarlan, James K. Fredrickson, Kenneth W. Minton, Min Zhai, Lawrence P. Wackett, and Michael J. Daly. “Engineering Deinococcus Radiodurans for Metal Remediation in Radioactive Mixed Waste Environments.” Nature Biotechnology 18, no. 1 (January 2000): 85–90. https://doi.org/10.1038/71986.
- Carrière, Yves, David W. Crowder, and Bruce E. Tabashnik. “Evolutionary Ecology of Insect Adaptation to Bt Crops.” Evolutionary Applications 3, no. 5-6 (April 30, 2010): 561–73. https://doi.org/10.1111/j.1752-4571.2010.00129.x.
- Dively, Galen P., P. Dilip Venugopal, Dick Bean, Joanne Whalen, Kristian Holmstrom, Thomas P. Kuhar, Hélène B. Doughty, Terry Patton, William Cissel, and William D. Hutchison. “Regional Pest Suppression Associated with Widespread Bt Maize Adoption Benefits Vegetable Growers.” Proceedings of the National Academy of Sciences 115, no. 13 (March 12, 2018): 3320–25. https://doi.org/10.1073/pnas.1720692115.
- Green, Kate. “Gene Editing Study Finds Gene for Heat Tolerance in Corals.” AIMS, 2021. https://www.aims.gov.au/information-centre/news-and-stories/gene-editing-study-finds-gene-heat-tolerance-corals.
- Hamdan, Mohd Fadhli, and Boon Chin Tan. “Genetic Modification Techniques in Plant Breeding: A Comparative Review of CRISPR/Cas and GM Technologies.” Horticultural Plant Journal, October 3, 2024. https://doi.org/10.1016/j.hpj.2024.02.012.
- Hutchison, W. D., E. C. Burkness, P. D. Mitchell, R. D. Moon, T. W. Leslie, S. J. Fleischer, M. Abrahamson, et al. “Areawide Suppression of European Corn Borer with Bt Maize Reaps Savings to Non-Bt Maize Growers.” Science 330, no. 6001 (October 7, 2010): 222–25. https://doi.org/10.1126/science.1190242.
- Innovative Genomics Institute (IGI). “CRISPR Technology,” January 17, 2025. https://innovativegenomics.org/crisprpedia/crispr-technology/#CRISPR-genome-editing.
- Kathage, Jonas, and Matin Qaim. “Economic Impacts and Impact Dynamics of Bt (Bacillus Thuringiensis) Cotton in India.” Proceedings of the National Academy of Sciences 109, no. 29 (July 2, 2012): 11652–56. https://doi.org/10.1073/pnas.1203647109.
- Kelley, Melissa L., Žaklina Strezoska, Kaizhang He, Annaleen Vermeulen, and Anja van Brabant Smith. “Versatility of Chemically Synthesized Guide RNAs for CRISPR-Cas9 Genome Editing.” Journal of Biotechnology 233 (September 2016): 74–83. https://doi.org/10.1016/j.jbiotec.2016.06.011.
- Lebedev, Vadim G., Anna A. Popova, and Konstantin A. Shestibratov. “Genetic Engineering and Genome Editing for Improving Nitrogen Use Efficiency in Plants.” Cells 10, no. 12 (December 1, 2021): 3303. https://doi.org/10.3390/cells10123303.
- Liu, Chang, Li Zhang, Hao Liu, and Kun Cheng. “Delivery Strategies of the CRISPR-Cas9 Gene-Editing System for Therapeutic Applications.” Journal of Controlled Release 266 (September 11, 2017): 17–26. https://doi.org/10.1016/j.jconrel.2017.09.012.
- McGorman, Cillian. “CRISPR-Cas9 Bioremediation | ERS Genomics.” ERS Genomics, June 21, 2024. https://ersgenomics.com/crispr-cas9-bioremediation/.
- McGovern Institute. “Genome Editing with CRISPR-Cas9.” YouTube, November 6, 2014. https://www.youtube.com/watch?v=2pp17E4E-O8.
- MedlinePlus. “What Are Genome Editing and CRISPR-Cas9?” Medlineplus. National Library of Medicine, March 22, 2022. https://medlineplus.gov/genetics/understanding/genomicresearch/genomeediting/.
- Nester, Eugene W., Linda S. Thomashow, Matthew Metz, and Milton Gordon. 100 Years of Bacillus Thuringiensis: A Critical Scientific Assessment: This Report Is Based on a Colloquium, “100 Years of Bacillis Thuringiensis, a Paradigm for Producing Transgenic Organisms: A Critical Scientific Assessment,” Sponsored by the American Academy of Microbiology and Held November 16–18, in Ithaca, New York. PubMed. Washington (DC): American Society for Microbiology, 2002. https://www.ncbi.nlm.nih.gov/books/NBK559445/.
- Rafeeq, Hamza, Nadia Afsheen, Sadia Rafique, Arooj Arshad, Maham Intisar, Asim Hussain, Muhammad Bilal, and Hafiz M.N. Iqbal. “Genetically Engineered Microorganisms for Environmental Remediation.” Chemosphere 310, no. 310 (January 2023): 136751. https://doi.org/10.1016/j.chemosphere.2022.136751.
- Romeis, Jörg, Steven E. Naranjo, Michael Meissle, and Anthony M. Shelton. “Genetically Engineered Crops Help Support Conservation Biological Control.” Biological Control 130 (March 2019): 136–54. https://doi.org/10.1016/j.biocontrol.2018.10.001.
- Salazar-García, Luis M, Luis C Damas-Ramos, Luisa M Trejo-Alarcón, Daniela Rago, Linda Ahonen, Pablo Cruz-Morales, Patricia Ponce-Noyola, and Cuauhtémoc Licona-Cassani. “CRISPR-Driven Enhanced Hydrocarbon Emulsification in an Environmental Pseudomonas Aeruginosa Strain.” Microbial Cell Factories 24, no. 1 (July 2, 2025). https://doi.org/10.1186/s12934-025-02769-y.
- The Pirbright Institute. “Genetic Engineering.” The Pirbright Institute, March 16, 2018. https://www.pirbright.ac.uk/engage-with-us/science-case-studies/genetic-engineering.
- Trafton, Anne. “New CRISPR-Based Tool Inserts Large DNA Sequences at Desired Sites in Cells.” MIT News | Massachusetts Institute of Technology, November 24, 2022. https://news.mit.edu/2022/crispr-gene-editing-dna-1124.
- Upholt, Boyce. “Inside the Quest to Engineer Climate-Saving ‘Super Trees.’” MIT Technology Review, June 8, 2023. https://www.technologyreview.com/2023/06/08/1074287/inside-the-quest-to-engineer-climate-saving-super-trees/.
- Wang, Chenfei, Chaolan Pan, Haiyang Yong, Feifei Wang, Tao Bo, Yitong Zhao, Bin Ma, Wei He, and Ming Li. “Emerging Non-Viral Vectors for Gene Delivery.” Journal of Nanobiotechnology 21, no. 1 (August 17, 2023): 272. https://doi.org/10.1186/s12951-023-02044-5.
- Wilson, Ross. “CRISPR Technology.” Innovative Genomics Institute (IGI), 2022. https://innovativegenomics.org/crisprpedia/crispr-technology/.
- Xie, Yun, Feng Yu, Qi Wang, Xin Gu, and Wuling Chen. “Cloning of Catechol 2,3-Dioxygenase Gene and Construction of a Stable Genetically Engineered Strain for Degrading Crude Oil.” Indian Journal of Microbiology 54, no. 1 (April 21, 2013): 59–64. https://doi.org/10.1007/s12088-013-0411-2.